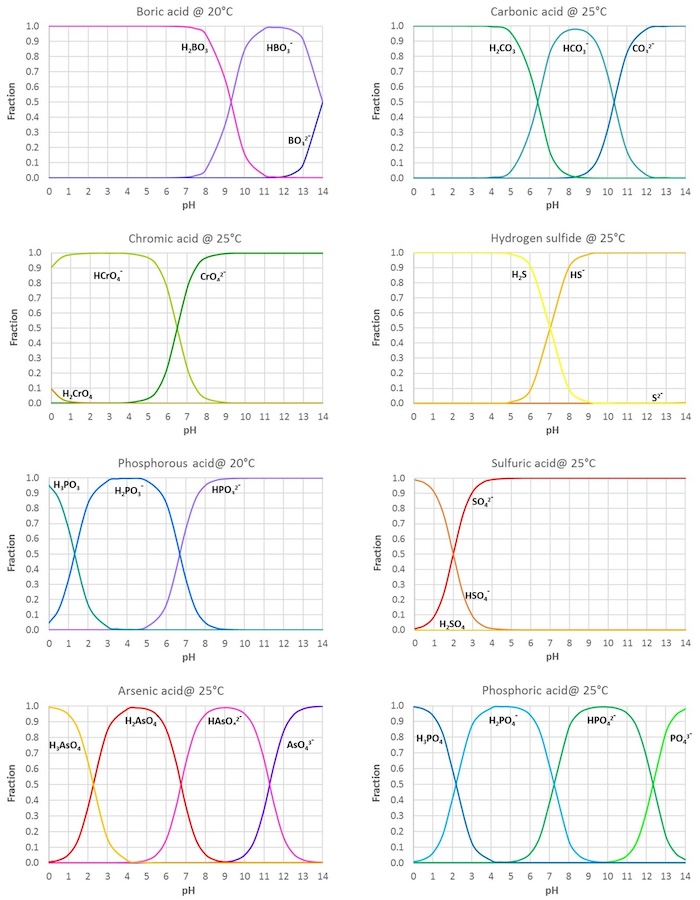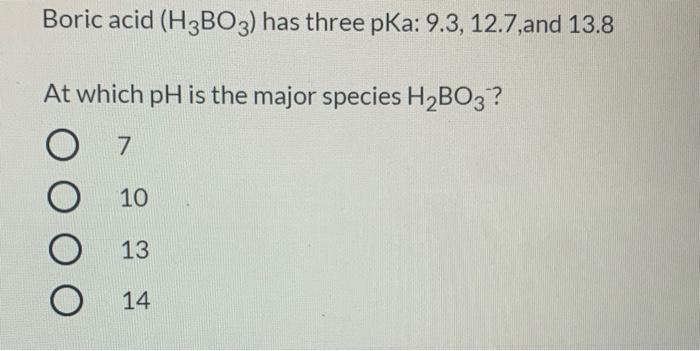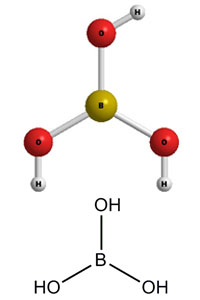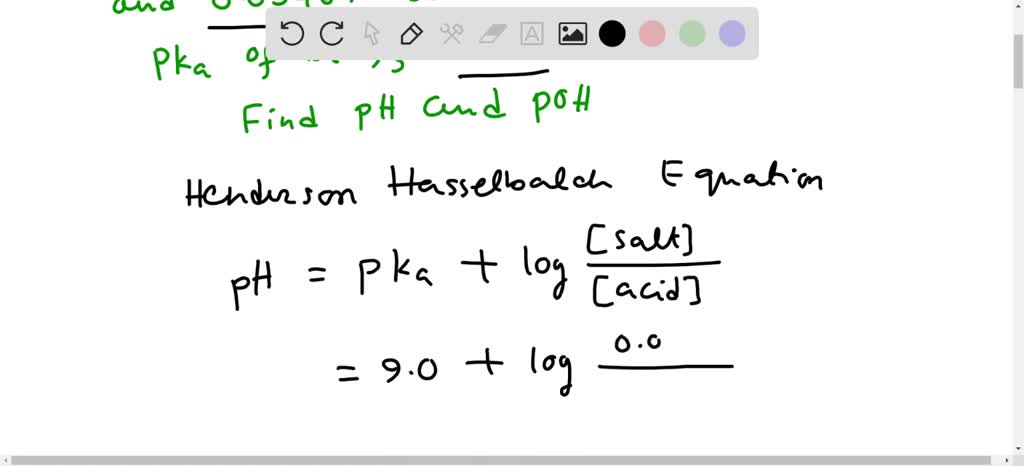
SOLVED: Determine the pH and pOH of 0.25 L of a solution that is 0.0226 M boric acid and 0.0346 M sodium borate; pKa for B(OH)3 = 9.0 at 25°C.

Boric Acid, a Lewis Acid With Unique and Unusual Properties: Formulation Implications - Journal of Pharmaceutical Sciences

Distribution of polyborate species as a function of pH in 0.4 M boric acid | Download Scientific Diagram
Method transfer assessment for boric acid assays according to different pharmacopoeias' monographs | SpringerLink

Origins, and formulation implications, of the pKa difference between boronic acids and their esters: A density functional theory study | Semantic Scholar
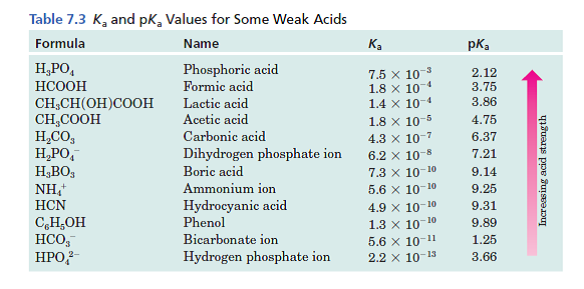
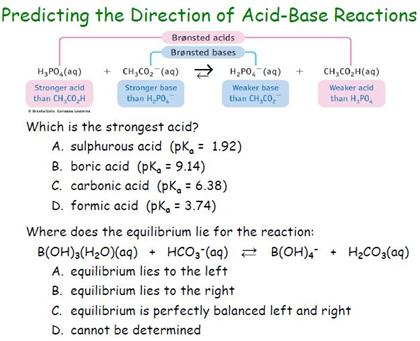
CHEM1902/4 2013-N-4 November 2013 • Boric acid, B(OH) 3, is a weak acid (pKa = 9.24) that is used as a mild antiseptic and

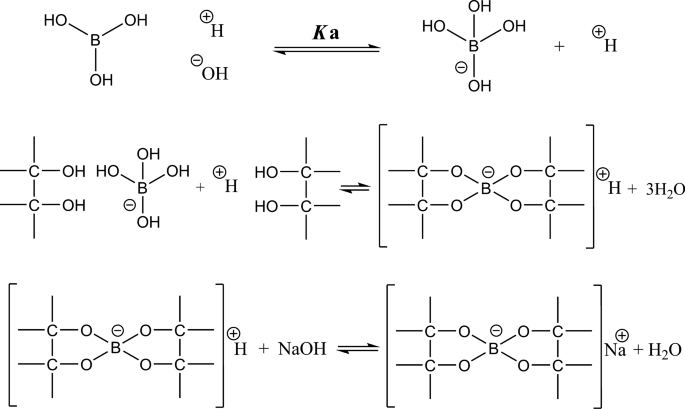
Boric Acid, a Lewis Acid With Unique and Unusual Properties: Formulation Implications - Journal of Pharmaceutical Sciences
![SOLVED: aqueous solution, boric acid behaves as a weak In acid 9.1) and the following = equilibrium (pKa is established: > [a B(OH)3(aq) + 2HzO() i Anillsn [B(OH)4]-(aq) + [H;o]t(aq) (a) Draw SOLVED: aqueous solution, boric acid behaves as a weak In acid 9.1) and the following = equilibrium (pKa is established: > [a B(OH)3(aq) + 2HzO() i Anillsn [B(OH)4]-(aq) + [H;o]t(aq) (a) Draw](https://cdn.numerade.com/ask_images/93cea8efb5744328b36ee05fe1813a8d.jpg)