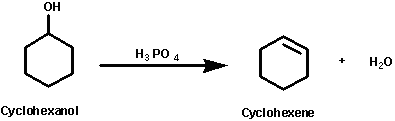![Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid. Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.](https://cdn1.byjus.com/wp-content/uploads/2018/11/phosphoric-acid-structure.png)
Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.

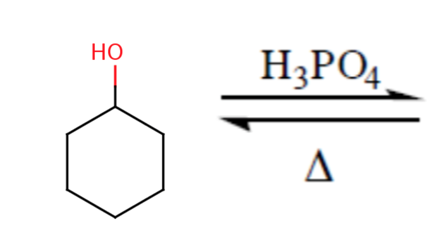
In the reaction of cyclohexanol and phosphoric acid to produce cyclohexene, would the Rf of the alcohol be greater or less than the cyclohexene if thin layer chromatography was done? Explain why.

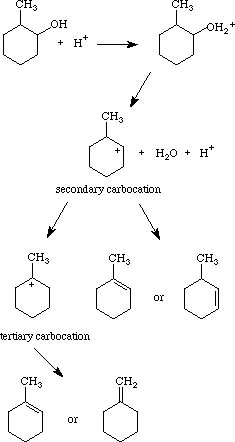
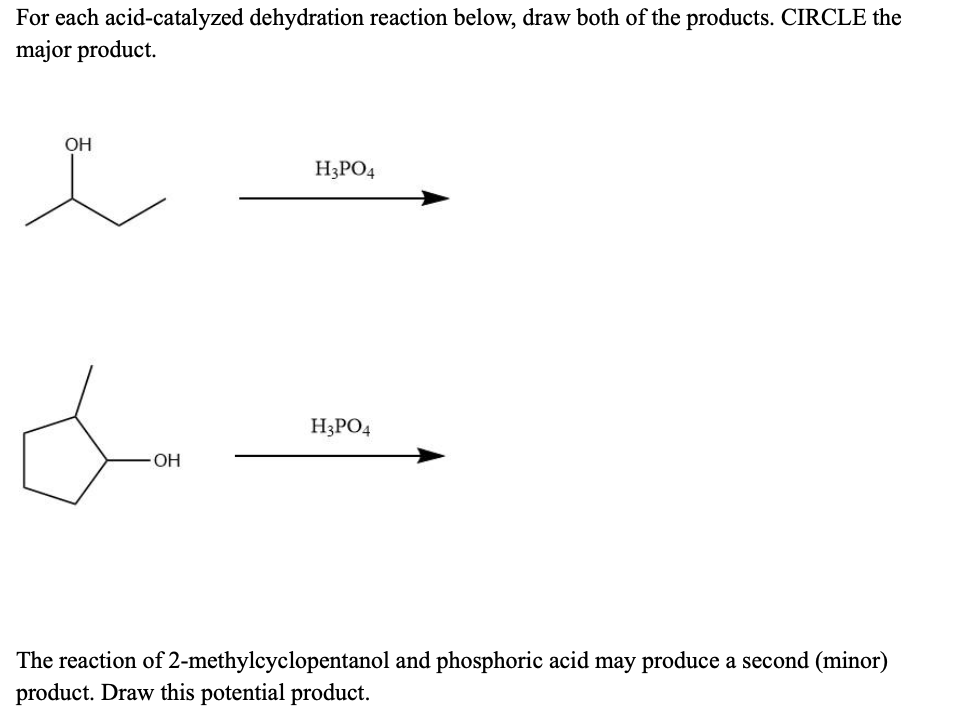
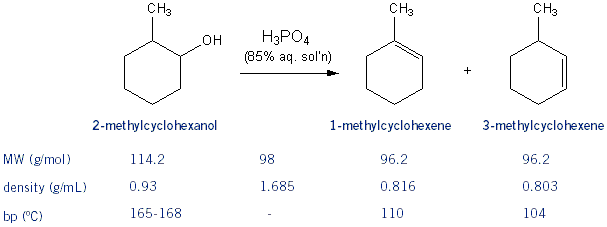
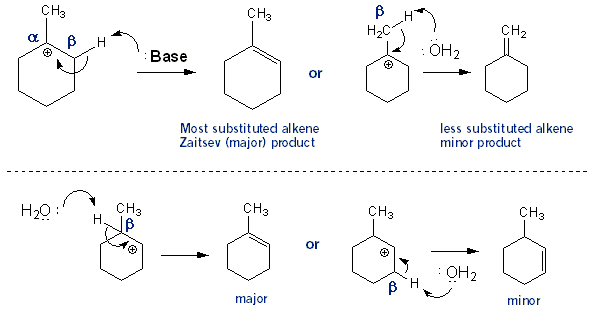
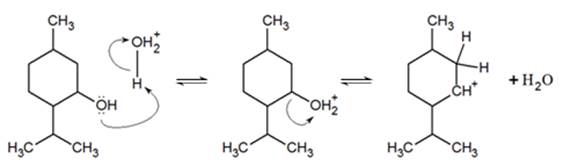
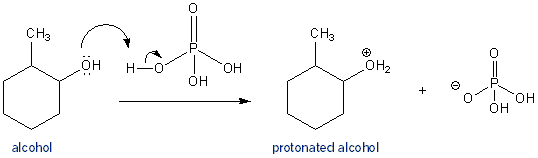
1) write the mechanism expected for the dehydration reaction of cyclohexanol with phospharic acid. 2) provide the major and minor products expected if 1-methylcyclohexanol, 2- methylcylcohexanol, 3- m | Homework.Study.com